Abstract
The gut microbiota is a fundamental regulator of host immune responses. Our group previously demonstrated that the depletion of the gut microbiota by broad-spectrum antibiotics (ABX) prevented neutrophil aging (a type of circadian priming) and vaso-occlusion and prolonged survival in a mouse model for sickle cell disease (SCD) (Zhang, D et al. Nature 2015, 570(7570):528-532); the mechanisms involved, however, are as yet unknown.
Diverse metabolites are generated by the microbiota from the fermentation of exogenous undigested dietary components and endogenous substrates secreted by the host. Complex carbohydrates are metabolized into short-chain fatty acids (SCFAs), while cholesterol derivatives from the liver are converted into secondary bile acids by intestinal bacteria. The major SCFA producers are Bacteroidetes (gram-negative) and Firmicutes (gram-positive) divisions, and dysbiosis of these bacterial divisions is found in both SCD humans and mice, (Lim, S et al. Am J Hematol 2018, 93(4):E91-E93).
Herein, we investigate whether and how microbiota-derived metabolites modulate the dynamics of neutrophils in vaso-occlusive processes in SCD mice. First, to C57BL/6 (wild-type, WT) mice we administered either: 1) one of three main SCFAs, namely sodium butyrate (Na-B), sodium propionate (Na-P), or sodium acetate (Na-A); or 2) a combination of two main secondary bile acids, lithocholic and deoxycholic acids. The number of circulating neutrophils with an aged phenotype (i.e., Ly6Ghi CD11bhi CD62Llo CXCR4pos, Casanova-Acebes, M et al. Cell 2013, 153(5):1025-1035) was assessed by flow cytometry over time. Numbers started to increase at 2h after Na-B administration and we observed a peak of accumulation of total and aged-like neutrophils at 4-5h, with a return to basal after 9h.
Consistent with this finding, administration of Na-B to SCD mice led to augmented counts of aged-like neutrophils and impaired survival of mice subjected to inflammation. Interestingly, when SCD mice receiving prolonged ABX treatment (40-45 days) were also given daily Na-B for the last days of ABX treatment, the percentage of aged-like neutrophils in the circulation was higher than that found in the blood of SCD mice under ABX, but without Na-B (55% vs 24%), showing that Na-B by itself is able to reverse the reduction in neutrophil aging promoted by ABX.
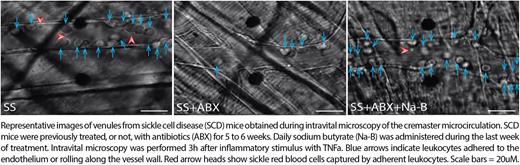
To address the impact of Na-B in SCD vaso-occlusion, intravital microscopy of the cremaster muscle of SCD mice, after inflammatory stimulus with TNFa, revealed that Na-B abrogated the beneficial effects of ABX and triggered vaso-occlusive processes. We found a 3-fold rise in the adhesion of leukocytes to the endothelium and a 2-fold slower blood flow rate in the microvasculature of mice who received Na-B (see Figure). These results support our hypothesis that the gut microbiota modulates inflammation and vaso-occlusion in SCD through the generation of SCFAs. Because Na-B is known to be a potent inhibitor of histone deacetylase (HDAC) enzymes, we induced HDAC inhibition in WT mice. Vorinostat, a potent synthetic HDAC inhibitor, led to a 3-fold greater expansion of total and aged-like neutrophils in the blood, suggesting a role for HDACs in neutrophil biology. Consistently, in vitro treatment of WT mouse neutrophils with vorinostat or Na-B accelerated the progressive acquisition of an aged-like phenotype, characterized by loss of L-selectin (CD62L).
Proteome of neutrophils from WT mice after induction of circadian aging by anti-P and anti-E-selectin antibodies showed that aged neutrophils are similar to neutrophils from vorinostat-treated mice, with down-regulation of genes involved in mRNA processing, protein synthesis, and proteasome complex formation, suggesting that HDAC inhibition is the underlying mechanism driving neutrophil aging. Importantly, the down- and up-regulated pathways found in these neutrophils were the same as those found in SCD mouse neutrophils, and an opposite proteomic profile was observed in neutrophils from ABX-treated SCD mice. Our findings indicate that the intestinal microbiota drives vaso-occlusion and neutrophil aging in SCD mice through the production of metabolites that inhibit HDAC. They also define the microbiota as instigator and innate immunity as effector of vascular disease, such that targeting their crosstalk could be effective at alleviating the devastating symptoms in SCD patients.
Disclosures
Hidalgo:Flagship Pioneering Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal